The Department of Medicine would like to congratulate our resident clinician innovators on their recent successful participation in the Georgia Tech/Emory Biomedical Engineering (BME) Capstone projects!
As part of the J. Willis Hurst Internal Medicine Residency Programs’ Distinction in Medical Innovation program, internal medicine residents successfully pitched relevant healthcare problems that were selected for medical device development by senior capstone BME teams this fall semester. Our residents served as key members of their teams, providing project leadership and key clinical guidance. The BME teams showcased their completed device solutions at the recent Georgia Tech Capstone Design Expo.
Georgia Tech students highly value the opportunity to work with Emory faculty and residents as they receive credentialing and access for clinical observations at Emory Healthcare and Grady Health System facilities through the Student Training for Advancing Technologies Credentialing program (STAT), co-sponsored by the dean’s office at Emory’s School of Medicine. This program has afforded the Georgia Tech students critical opportunities to understand the need and the environments in which their innovations will be implemented.
View our residents’ projects and videos below:
Ronnie Funk, MD (Clinical Mentor)
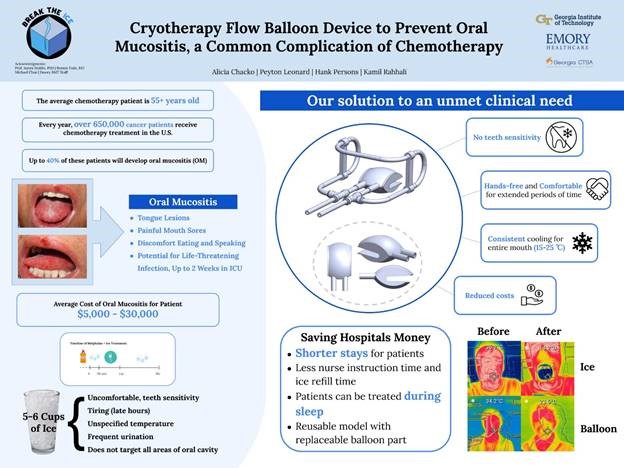
“Cryotherapy Flow Balloon Device to Prevent Oral Mucositis, a Common Complication of Chemotherapy”
Break the Ice BME Team Members:
- Alicia Chacko — Rome, GA
- Henry Persons — Atlanta, GA
- Kamil Rahhali — Weehawken, NJ
- Peyton Leonard — Brookhaven, GA
Project Description: We are building a cryotherapy flow device to prevent oral mucositis, a side effect of chemotherapy. Oral mucositis is a serious and debilitating disease that impacts up to forty percent of chemotherapy patients. Chemotherapy attacks rapidly dividing cells lining the mouth, resulting in painful lesions and mouth sores. This can not only affect a patient’s speech and nutrition, but higher grades of oral mucositis can also lead to life-threatening, potentially fatal, infections for these immunocompromised patients. The current prevention method is eating ice before, during, and after the chemotherapy infusion. The major problems with this treatment are inconsistent and uneven temperature distribution, lack of hands-free use, and discomfort, especially from contact of ice with sensitive areas such as teeth. Our solution is the CryoBalloon. It is a cryotherapy flow device that circulates ice-cold saline or water through a balloon structure to cool the inside of the mouth. The design allows for even temperature distribution, hands-free use, and limited teeth sensitivity. The CryoBalloon lowers the temperature of the inside of the mouth below 25 degrees Celsius, promoting vasoconstriction of the blood vessels, which prevents the destruction of the inner lining of the mouth. This is how our device aids in preventing oral mucositis.
Sarah Kamal, MD (Clinical Mentor)
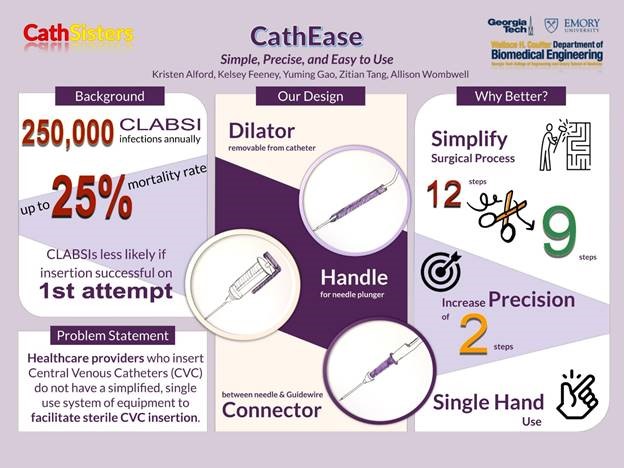
“CathEase: Simple, Precise, and Easy to Use”
Cathsisters BME Team Members:
- Allison Wombwell — Kansas City, MO
- Kelsey Feeney — Chicago, IL
- Kristen Alford — Jefferson, GA
- Yuming Gao — Beijing, China
- Zitian Tang — Beijing, China
Project Description: Redesigned central line catheter insertion device to minimize cannulation attempts and improve patient outcomes. Placement of central venous catheters (CVC) is required for nearly all patients admitted to the Intensive Care Unit (ICU). They are required for the administration of essential medications in a quick and safe manner, and allow for continuous or frequent monitoring of important hemodynamic properties to better manage a patient with a shock syndrome. The placement of a CVC, however, also introduces a source for systemic bloodstream infection, a phenomenon known as central line-associated bloodstream infection (CLABSI). Hospitals have already implemented many policies to reduce CLABSI incidence with some success, although thousands of preventable infections still occur every year. Several factors have been associated with a higher incidence of CLABSI and have been the targets of these initiatives. A major factor that has been identified is the use of a sterile technique with the initial insertion of the catheter. To accomplish this, sterile drapes and gloves are used for the procedure. However, placement of a CVC is a multi-step process that can vary in duration depending on the ease of access and skill of the provider. For example, there is a step where you must enter the blood vessel with a needle connected to a syringe (see image below), and then twist off the syringe to introduce the guidewire for the catheter. If the needle is not far enough into the vessel, or if the provider’s hand slips while unscrewing the syringe, then the guidewire will not pass freely. In this case, you would have to withdraw the wire, re-attach the syringe, and attempt to reposition with possible repeat puncturing of the vessel. The more attempts, the more contact the provider will have to make with the patient and the more opportunities to break sterility. Patients whose CVCs are placed after multiple attempts are more likely to develop an infection than those who only required one attempt. Therefore, re-designing the equipment used to insert the catheter with the goal of reducing the number of cannulation attempts could significantly impact the incidence of CLABSI. In the United States, over 100,000 CLABSIs occur annually with an estimated mortality rate of 10 to 30 percent. In addition to the added costs of prolonged hospitalization and treatment associated with a CLABSI, the Centers for Medicare and Medicaid Services administer financial penalties to hospitals with higher CLABSI rates. Given the high financial incentive, hospitals have invested millions in research and initiatives to lower CLABSI incidence. Reduction in CLABSI occurrence would not only improve patient outcomes but also greatly reduce costs to the hospital system.
Sophie Vitter, MD (Clinical Mentor)
“Edema of the State: Standardized Edema Gauge”
Edema of the State BME Team Members:
- Bencharles Okolie — Jonesboro, GA
- Gwyneth Pokallus — Milton, GA
- Julia Behrend — Alpharetta, GA
- Stella Nantale — Fairburn, GA
Project Description: Standardized metric for measuring lower extremity Edema. Lower extremity edema is an indication that there is fluid excess in the interstitial tissues of congestive heart failure patients. Constant measuring and assessing the extent of the edema is crucial and necessary. The current metric is subjective and non-standardized since it varies from one clinician to another. This causes discrepancies in the patient documentation and care. The goal is to create a standardized metric for measuring lower extremity edema. A handheld device that measures change in skin depth and times the skin elasticity.
Watch Edema of the State Video
Krishan Patel, MD (Clinical Mentor)
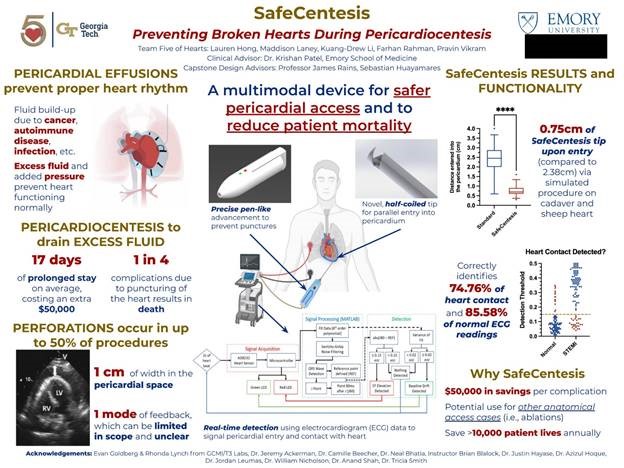
“SafeCentesis: Preventing Broken Hearts During Pericardiocentesis”
Five of Hearts BME Team Members:
- Farhan Rahman — Suwanee, GA
- Kuang-Drew Li — Johns Creek, GA
- Lauren Hong — Alpharetta, GA
- Maddison Laney — Hoover, AL
- Pravin Vikram — Suwanee, GA
Project Description: Excess fluid can fill the pericardial sac – a fibrous, protective membrane around the heart – due to a multitude of diseases, including viral infections, autoimmune diseases, and cancer. This condition is known as a pericardial effusion, and in severe cases can prevent the heart from beating as in the case of cardiac tamponade. Physicians can perform a pericardiocentesis by inserting a large 18-gauge needle through the patient’s chest and into the pericardial space to drain this fluid. However, in up to 50 percent of pericardiocentesis procedures, the needle unintentionally perforates the vascular heart, and one in four people die due to this complication. Complications due to myocardial perforations can extend a patient’s hospital stay by seventeen days on average, costing tens of millions of dollars in excess costs to the US healthcare system. Team Five of Hearts devised the SafeCentesis, a novel approach that allows physicians to safely access the pericardial space by mechanically entering the pericardium no more than 1cm, engaging a half-coiled needle tip for parallel insertion into the heart to reduce risk of puncture, and providing real-time feedback to signal the needle’s relative position to the heart. Our device presents a vast opportunity to save lives and create value in a market of $64 million in the United States alone. With the help of SafeCentesis, Team Five of Hearts can save weeks of prolonged, painful hospital stays and reduce costs by more than $50,000 per puncture. Our device offers to save over 10,000 patients annually, which not only creates a safer environment for patients but also increases physician confidence and enables further, more advanced treatments in the pericardial space and treating other anatomical access cases.
Omid Behbahani-Nejad, MD (Clinical Mentor)
“The JVPro: Jugular Venous Pressure Waveform Detection Device”
JVPros BME Team Members:
- Akshaya Venkata — Land O Lakes, FL
- Brock Miller — Marietta, GA
- David Espinal — Miami, FL
- Naseem Sadek — Tucker, GA
- Samantha Rodriguez — Miami, FL
Project Description: Revitalizing how we evaluate jugular venous pressure. Measuring jugular venous pressure (JVP) is an important clinical skill that unfortunately feels somewhat archaic in an era where medical innovation is prospering. The skill of measuring JVP requires a significant amount of repetition to master because of the timely process it takes to appreciate the contours of the neck. Despite having observed the spectrum of different jugular venous pressures, reported findings of jugular venous pressures can often be imprecise because it is clinician-dependent. Background: Jugular venous pressure is often thought to be the hallmark of whether a patient is volume-overloaded in terms of clinical status. There are other markers that can help supplement the clinical gestalt in judging a patient’s current volume status (i.e., physical exam findings such as cardiopulmonary abnormalities, abdominal distention, and edema in the lower extremities). However, JVP is often emphasized in academic hospitals as the defining factor that influences a patient’s treatment plan because managing it can often have a dramatic effect on a patient’s active symptoms. Target population: Cardiac patients, such as those with heart failure with preserved ejection fraction, heart failure with reduced ejection fraction, end-stage heart failure supported by ventricular assist devices, patients with valvular heart disease, or those with tamponade physiology. Clinical vision: JVPro is a device that any healthcare member could attach comfortably to a patient (non-invasively) and automatically calculate the JVP.
Lilas Dagher, MD (Clinical Mentor)
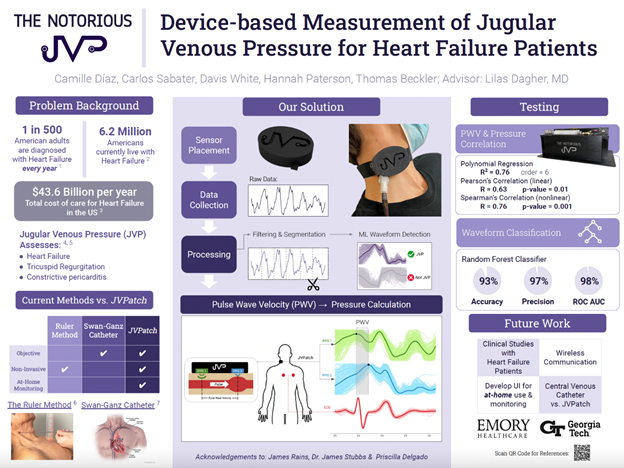
“Device-based Measurement of Jugular Venous Pressure for Heart Failure Patients”
The Notorious J.V.P. BME Team Members:
- Camille Diaz — San Juan, PR
- Carlos Sabater — San Juan, PR
- Davis White — Alpharetta, GA
- Hannah Paterson — Westwood, MA
- Thomas Beckler — Milton, GA
Project Description: Heart Failure (HF) affects over 6.8 million people in the United States and accounts for over one million annual hospitalizations. Treating HF costs the U.S. around $43.6 billion annually. Hypervolemia is an indicator of HF and is assessed by measuring jugular venous pressure (JVP). The current method to assess JVP involves measuring the height of the peak pulsation in the internal jugular vein (IJV) above the sternal angle using rulers. This method is subject to high inter-observer and intra-user variability. Physicians disregard this method and instead opt for visual descriptions of the JVP, which further increases inter-observer variability. While other quantitative and non-invasive JVP sensors have been made, these rely on experts to locate the IJV for placement. Locating the IJV is difficult, even for advanced HF experts. There is a need to provide an objective JVP measurement in a way that decreases inter-observer variability and does not require expert placement. Our team created a wearable device consisting of multiple photoplethysmography (PPG) sensors aided by an electrocardiogram (ECG) to automatically locate the IJV and determine JVP. Each sensor determines if the jugular venous pressure waveform is present. Using the identified JVP waveforms, we can determine the pulse wave velocity (PWV) of blood in the IJV, which can be used to approximate the JVP. We expect that this approach will provide physicians with a reliable, objective measurement of JVP, with the potential for at-home monitoring. This would allow physicians to track patients’ condition and adjust their medication before they experience HF decompensation and are re-hospitalized.
Sophie Vitter, MD (Clinical Mentor)
“YouRN Good Hands”
“YouRN Good Hands” BME Team Members:
- Brandon Dobson — Sharpsburg, GA
- Caroline Means — Suwanee, GA
- David Frey Rubio — Dallas, GA
- Liya Woldye — Shashemene, Ethiopia
- Yujin Choi — Seoul, Korea
Project Description: Urine Output Measurement for Congestive Heart Failure Patients. Approximately 6.2 million Americans yearly suffer from Heart Failure. Due to decreased blood flow, kidneys are unable to keep up with the demands of the body, leading to the body retaining fluid and swelling of the limbs. To help treat this, patients are prescribed diuretics. The dosages for these diuretics must be regulated to ensure a safe net loss of body fluid. Urine output measurements are critical to determining a patient’s proper diuretic dosage. However, due to a lack in patient compliance and high workload for attending nurses, these measurements can be inaccurate. The purpose of our project is to improve the urine output measurement process’ reliability and accuracy to help deliver proper diuretic dosage, and lessen patient hospital stays.





Be the first to comment on "Internal medicine residents collaborate with biomedical engineering students on Georgia Tech/Emory Biomedical Engineering (BME) Senior Capstone projects"